Site-selective C–H alkylation of myo-inositol via organic photoredox catalysis†
Abstract
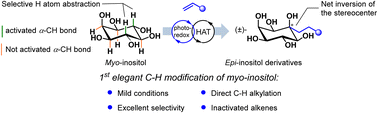
Site-selective photoredox reactions with aromatic olefins enable direct alkylation of unprotected myo-inositol at C4. The efficacy of these reactions can be finely tuned by modifying the structures of HAT reagents. These reactions open the possibility of selective C–H alkylations of myo-inositol without the need for multi-step protection–deprotection strategies.


 Please wait while we load your content...
Please wait while we load your content...