Total synthesis of (−)-panduratin D†
Abstract
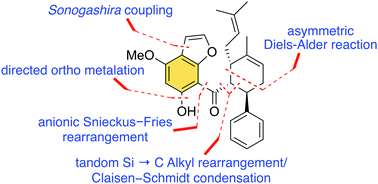
Herein, an enantioselective total synthesis of (−)-panduratin D, a novel secondary metabolite against human pancreatic PANC-1 cancer cell, from commercially available 3-methoxyphenol is reported. The synthesis was completed in nine steps and the key features include Sonogashira coupling, anionic Snieckus–Fries rearrangement, directed ortho metalation, tandem Si → C Alkyl rearrangement/Claisen–Schmidt condensation, and chiral boron complex-promoted asymmetric Diels–Alder cycloaddition. These endeavors could facilitate the biological studies of (−)-panduratin D and its analogs.


 Please wait while we load your content...
Please wait while we load your content...