l-Proline, a resolution agent able to target both enantiomers of mandelic acid: an exciting case of stoichiometry controlled chiral resolution†
Abstract
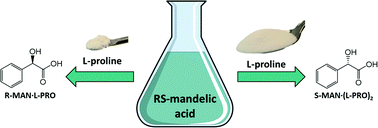
We present a thought-provoking development in chiral resolution. Using a resolving agent of a given handedness, L-proline, we show that both R- and S-enantiomers of mandelic acid can be resolved from a racemic mixture simply by varying the stoichiometry. We are the first to report this specific feature, achieved by the existence of stoichiometrically diverse cocrystal systems between R- and S-mandelic acid and L-proline.


 Please wait while we load your content...
Please wait while we load your content...