Stereoselective insertion of cyclopropenes into Mg–Mg bonds†
Abstract
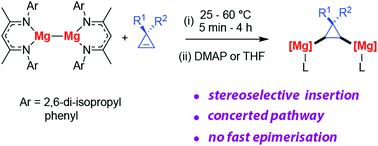
The reaction of cyclopropenes with compounds containing Mg–Mg bonds is reported. 1,2-Dimagnesiation occurs exclusively by syn-addition to the least hindered face of the alkene forming a single diastereomeric product. DFT calculations support a concerted and stereoselective mechanism. These findings shed new light on the stereochemistry of reactions involving magnesium reagents.


 Please wait while we load your content...
Please wait while we load your content...