Catalytic asymmetric addition to cyclic N-acyl-iminium: access to sulfone-bearing contiguous quaternary stereocenters†
Abstract
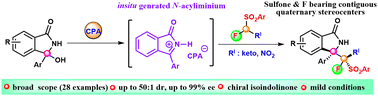
Herein, we report the first chiral phosphoric acid (CPA)-catalyzed asymmetric addition of α-fluoro(phenylsulfonyl)methane (FSM) derivatives to in situ generated cyclic N-acyliminium. This process enables metal-free expeditious access to sulfone and fluorine incorporating contiguous all substituted quaternary stereocenters ingrained in biorelevant isoindolinones in excellent stereoselectivities (up to 99% ee and up to 50 : 1 dr).


 Please wait while we load your content...
Please wait while we load your content...