A short total synthesis of (±)-mersicarpine via visible light-induced cascade photooxygenation†
Abstract
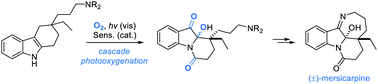
A short total synthesis of the Kopsia alkaloid (±)-mersicarpine is presented. As the key step, a visible light-induced catalytic cascade photooxygenation was utilized, to convert a 3,3-disubstituted tetrahydrocarbazole intermediate, in one step, into a perhydropyrido[1,2-a]indole dione as the immediate precursor to the natural product. The synthesis of mersicarpine was achieved with an overall yield of 12% over 13 steps.


 Please wait while we load your content...
Please wait while we load your content...