Turn-on mode fluorescent diarylethene containing neopentyl substituents that undergoes all-visible-light switching†
Abstract
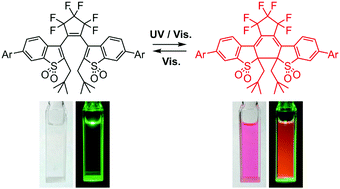
This paper presents a strategy for improving the all-visible-light switching response of turn-on mode fluorescent diarylethene derivatives. Introduction of neopentyl or isobutyl substituents at the reactive carbons (2- and 2′-positions) of an oxidized bis(benzothienyl)perfluorocyclopentene derivative, which undergoes both cyclization and cycloreversion reactions upon irradiation with visible light, was effective in increasing the cycloreversion quantum yield by one or two orders of magnitude in comparison with the yield of an ethyl-substituted derivative. Any significant influence on the cyclization and fluorescence quantum yields was not observed by the introduction of neopentyl or isobutyl substituents.


 Please wait while we load your content...
Please wait while we load your content...