Metal-free synthesis of gem-difluorinated heterocycles from enaminones and difluorocarbene precursors†
Abstract
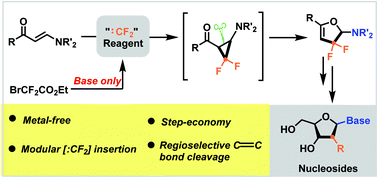
A cascade strategy to synthesise gem-difluorinated 2H-furans from reactions of BrCF2CO2Et with enaminones has been described. The reactions tolerate a wide variety of functional groups under metal-free conditions. An active aminocyclopropane is proposed to be a key intermediate through the cyclopropanation of difluorocarbene with enaminones, which further triggers a regioselective C–C bond cleavage in situ to afford the corresponding gem-difluorinated 2H-furans.


 Please wait while we load your content...
Please wait while we load your content...