Synthesis of polycyclic aminal heterocycles via decarboxylative cyclisation of dipeptide derivatives†
Abstract
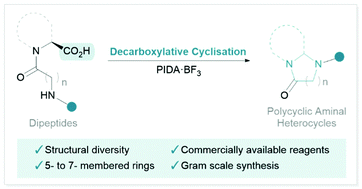
An oxidative-decarboxylative intramolecular cyclisation of dipeptide derivatives is reported. This transformation is promoted by phenyl iodine(III) diacetate (PIDA) in combination with BF3·OEt2. The reaction gives access to a variety of valuable polycyclic N-heterocyclic scaffolds containing 5-, 6-, or 7-membered rings.
- This article is part of the themed collection: 2022 Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...