R-Group stabilization in methylated formamides observed by resonant inelastic X-ray scattering†
Abstract
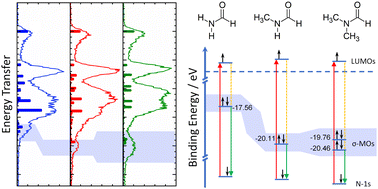
The inherent stability of methylated formamides is traced to a stabilization of the deep-lying σ-framework by resonant inelastic X-ray scattering at the nitrogen K-edge. Charge transfer from the amide nitrogen to the methyl groups underlie this stabilization mechanism that leaves the aldehyde group essentially unaltered and explains the stability of secondary and tertiary amides.


 Please wait while we load your content...
Please wait while we load your content...