Direct asymmetric reductive amination of α-keto acetals: a platform for synthesizing diverse α-functionalized amines†
Abstract
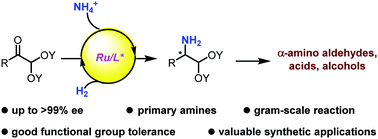
We report an efficient and straightforward method to synthesize enantio-enriched N-unprotected α-amino acetals via ruthenium-catalyzed direct asymmetric reductive amination. The α-amino acetal products are versatile and valuable platform molecules that can be converted to the corresponding α-amino acids, amino alcohols, and other derivatives by convenient transformations.


 Please wait while we load your content...
Please wait while we load your content...