Reversal of regioselectivity in reactions of donor–acceptor cyclopropanes with electrophilic olefins†
Abstract
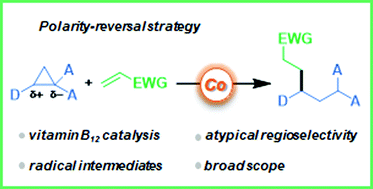
Reactivity of donor–acceptor cyclopropanes towards nucleophiles and electrophiles is determined by the specific philicity of the carbon atoms originating from the strong polarization of the central C–C bond. Herein, we report that vitamin B12 catalysis enables the transformation of an initially electrophilic center into a nucleophilic radical that reacts with SOMOphiles. This radical-based strategy reverses the standard regioselectivity and thus complements the classical approaches.


 Please wait while we load your content...
Please wait while we load your content...