Indole-substituted flavonol-based cysteine fluorescence sensing and subsequent precisely controlled linear CO liberation†
Abstract
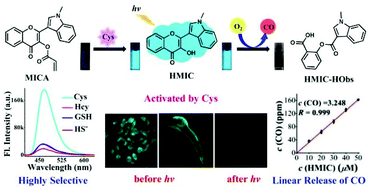
The first water-soluble B-ring-indole-substituted flavonol-based cysteine (Cys) fluorescent probe, MICA (2-(1-methyl-1H-indol-3-yl)-4-oxo-4H-chromen-3-yl-acrylate), was developed, which simultaneously serves as a precursor of photoCORM. In PBS buffer (only 15% DMF), MICA can perform rapid (330 s), highly chemoselective (particularly for homocysteine and glutathione) and sensitive (limit of detection: 92 nM) sensing and visualization of exogenous and endogenous Cys in live HeLa cells and zebrafish over a wide linear concentration range (0–12 μM/2.4 equiv.). The fluorophore HMIC (3-hydroxy-2-(1-methyl-1H-indol-3-yl)-4H-chromen-4-one), actuated and quantitatively generated via the sensing reaction of the precursor MICA with Cys, was designed as a photoCORM. By modulating the light illumination intensity or illumination duration or photoCORM dosage, HMIC can provide precisely controlled quantitative and linear CO gas by visible light illumination in aerobic environments. For live HeLa cells, MICA and all reaction products showed low toxicity (over 85% cell viability versus 10 μM analyst) and efficient cellular uptake. In live HeLa cells and zebrafish, both exogenous and endogenous Cys can be visualized by MICA, and the location and CO liberation process of the generated HMIC can be tracked in real time through its fluorescence. Substitution of the B-ring of 3-hydroxy-flavone (3-FL) by indole results in a 52 nm absorption red-shift vs.3-FL. Our work is the first water-soluble B-ring-indole-substituted flavonol-based fluorescent probe that efficaciously detects and visualizes exogenous and endogenous Cys both in vitro and in vivo, simultaneously serving as a precursor of photoCORM, actuated by Cys and triggered by visible light, releasing linear CO in aerobic environments. This work not only provides promising applications for the detection and visualization of exogenous and endogenous Cys, and spatiotemporally controllable CO liberation in live systems, but will also facilitate the development of handy molecular tools for clinical diagnosis and CO gas therapy.


 Please wait while we load your content...
Please wait while we load your content...