Illuminating anti-hydrozirconation: controlled geometric isomerization of an organometallic species†‡
Abstract
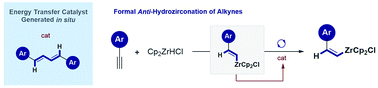
A general strategy to enable the formal anti-hydrozirconation of arylacetylenes is reported that merges cis-hydrometallation using the Schwartz Reagent (Cp2ZrHCl) with a subsequent light-mediated geometric isomerization at λ = 400 nm. Mechanistic delineation of the contra-thermodynamic isomerization step indicates that a minor reaction product functions as an efficient in situ generated photocatalyst. Coupling of the E-vinyl zirconium species with an alkyne unit generates a conjugated diene: this has been leveraged as a selective energy transfer catalyst to enable E → Z isomerization of an organometallic species. Through an Umpolung metal–halogen exchange process (Cl, Br, I), synthetically useful vinyl halides can be generated (up to Z : E = 90 : 10). This enabling platform provides a strategy to access nucleophilic and electrophilic alkene fragments in both geometric forms from simple arylacetylenes.


 Please wait while we load your content...
Please wait while we load your content...