Assembly of multicyclic isoquinoline scaffolds from pyridines: formal total synthesis of fredericamycin A†
Abstract
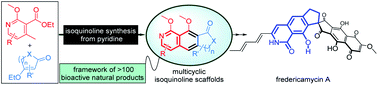
The construction of an isoquinoline skeleton typically starts with benzene derivatives as substrates with the assistance of acids or transition metals. Disclosed here is a concise approach to prepare isoquinoline analogues by starting with pyridines to react with β-ethoxy α,β-unsaturated carbonyl compounds under basic conditions. Multiple substitution patterns and a relatively large number of functional groups (including those sensitive to acidic conditions) can be tolerated in our method. In particular, our protocol allows for efficient access to tricyclic isoquinolines found in hundreds of natural products with interesting bioactivities. The efficiency and operational simplicity of introducing structural complexity into the isoquinoline frameworks can likely enable the collective synthesis of a large set of natural products. Here we show that fredericamycin A could be obtained via a short route by using our isoquinoline synthesis as a key step.


 Please wait while we load your content...
Please wait while we load your content...