Photocatalytic deoxygenation of N–O bonds with rhenium complexes: from the reduction of nitrous oxide to pyridine N-oxides†
Abstract
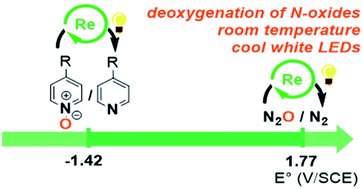
The accumulation of nitrogen oxides in the environment calls for new pathways to interconvert the various oxidation states of nitrogen, and especially their reduction. However, the large spectrum of reduction potentials covered by nitrogen oxides makes it difficult to find general systems capable of efficiently reducing various N-oxides. Here, photocatalysis unlocks high energy species able both to circumvent the inherent low reactivity of the greenhouse gas and oxidant N2O (E0(N2O/N2) = +1.77 V vs. SHE), and to reduce pyridine N-oxides (E1/2(pyridine N-oxide/pyridine) = −1.04 V vs. SHE). The rhenium complex [Re(4,4′-tBu-bpy)(CO)3Cl] proved to be efficient in performing both reactions under ambient conditions, enabling the deoxygenation of N2O as well as synthetically relevant and functionalized pyridine N-oxides.


 Please wait while we load your content...
Please wait while we load your content...