Catalyst-free arylation of sulfonamides via visible light-mediated deamination†
Abstract
A novel arylation of sulfonamides with boronic acids to afford numerous diaryl sulfones via a visible light-mediated N–S bond cleavage other than the typical transition-metal-catalyzed C(O)–N bond activation is described. This methodology, which represents the first catalyst-free protocol for the sulfonylation of boronic acids, is characterized by its simple reaction conditions, good functional group tolerance and high efficiency. Several successful examples for the late-stage functionalization of diverse sulfonamides indicate the high potential utility of this method in pharmaceutical science and organic synthesis.
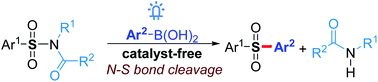


 Please wait while we load your content...
Please wait while we load your content...