A palladium-catalyzed approach to allenic aromatic ethers and first total synthesis of terricollene A†
Abstract
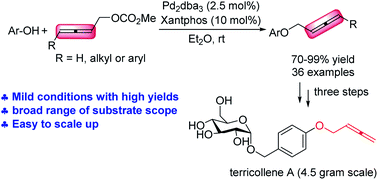
A palladium-catalyzed C–O bond formation reaction between phenols and allenylic carbonates to give 2,3-allenic aromatic ethers with decent to excellent yields under mild reaction conditions has been described. A variety of synthetically useful functional groups are tolerated and the synthetic utility of this method has been demonstrated through a series of transformations of the allene moiety. By applying this reaction as the key step, the total syntheses of naturally occurring allenic aromatic ethers, eucalyptene and terricollene A (first synthesis; 4.5 g gram scale), have been accomplished.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...