Abstract
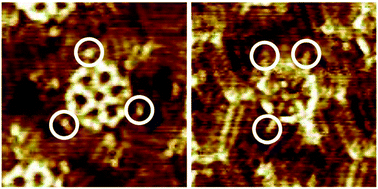
Molecular spoked wheels with D3h and Cs symmetry are synthesized by Vollhardt trimerization of C2v-symmetric dumbbell structures with central acetylene units and subsequent intramolecular ring closure. Scanning tunneling microscopy of the D3h-symmetric species at the solid/liquid interface on graphite reveals triporous chiral honeycomb nanopatterns in which the alkoxy side chains dominate the packing over the carboxylic acid groups, which remain unpaired. In contrast, Cs-symmetric isomers partially allow for pairing of the carboxylic acids, which therefore act as a probe for the reduced alkoxy chain nanopattern stabilization. This observation also reflects the adsorbate substrate symmetry mismatch, which leads to an increase of nanopattern complexity and unexpected templating of alkoxy side chains along the graphite armchair directions. State-of-the-art GFN-FF calculations confirm the overall structure of this packing and attribute the unusual side-chain orientation to a steric constraint in a confined environment. These calculations go far beyond conventional simple space-filling models and are therefore particularly suitable for this special case of molecular packing.


 Please wait while we load your content...
Please wait while we load your content...