Mechanochemical generation of acid-degradable poly(enol ether)s†
Abstract
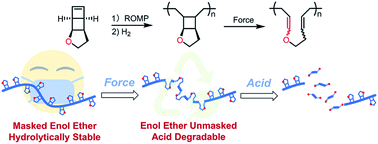
In an effort to develop polymers that can undergo extensive backbone degradation in response to mechanical stress, we report a polymer system that is hydrolytically stable but unmasks easily hydrolysable enol ether backbone linkages when force is applied. These polymers were synthesized by ring-opening metathesis polymerization (ROMP) of a novel mechanophore monomer consisting of cyclic ether fused bicyclohexene. Hydrogenation of the resulting polymers led to significantly enhanced thermal stability (Td > 400 °C) and excellent resistance toward acidic or basic conditions. Solution ultrasonication of the polymers resulted in up to 65% activation of the mechanophore units and conversion to backbone enol ether linkages, which then allowed facile degradation of the polymers to generate small molecule or oligomeric species under mildly acidic conditions. We also achieved solid-state mechano-activation and polymer degradation via grinding the solid polymer. Force-induced hydrolytic polymer degradability can enable materials that are stable under force-free conditions but readily degrade under stress. Facile degradation of mechanically activated polymechanophores also facilitates the analysis of mechanochemical products.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...