A novel catalytic heme cofactor in SfmD with a single thioether bond and a bis-His ligand set revealed by a de novo crystal structural and spectroscopic study†
Abstract
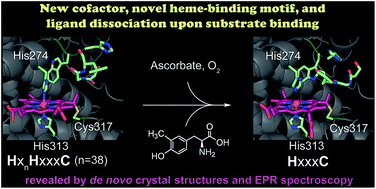
SfmD is a heme-dependent enzyme in the biosynthetic pathway of saframycin A. Here, we present a 1.78 Å resolution de novo crystal structure of SfmD, which unveils a novel heme cofactor attached to the protein with an unusual HxnHxxxC motif (n ∼ 38). This heme cofactor is unique in two respects. It contains a single thioether bond in a cysteine–vinyl link with Cys317, and the ferric heme has two axial protein ligands, i.e., His274 and His313. We demonstrated that SfmD heme is catalytically active and can utilize dioxygen and ascorbate for a single-oxygen insertion into 3-methyl-L-tyrosine. Catalytic assays using ascorbate derivatives revealed the functional groups of ascorbate essential to its function as a cosubstrate. Abolishing the thioether linkage through mutation of Cys317 resulted in catalytically inactive SfmD variants. EPR and optical data revealed that the heme center undergoes a substantial conformational change with one axial histidine ligand dissociating from the iron ion in response to substrate 3-methyl-L-tyrosine binding or chemical reduction by a reducing agent, such as the cosubstrate ascorbate. The labile axial ligand was identified as His274 through redox-linked structural determinations. Together, identifying an unusual heme cofactor with a previously unknown heme-binding motif for a monooxygenase activity and the structural similarity of SfmD to the members of the heme-based tryptophan dioxygenase superfamily will broaden understanding of heme chemistry.


 Please wait while we load your content...
Please wait while we load your content...