Synthesis of azahelicenes through Mallory reaction of imine precursors: corannulene substrates provide an exception to the rule in oxidative photocyclizations of diarylethenes†
Abstract
Typically, the synthesis of phenanthrene-based polycyclic aromatic hydrocarbons relies on the Mallory reaction. In this approach, stilbene (PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)-based precursors undergo an oxidative photocyclization reaction to join the two adjacent aromatic rings into an extended aromatic structure. However, if one C
CHPh)-based precursors undergo an oxidative photocyclization reaction to join the two adjacent aromatic rings into an extended aromatic structure. However, if one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C carbon atom is replaced by a nitrogen atom (C
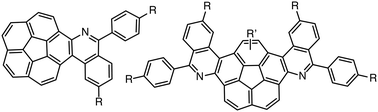
C carbon atom is replaced by a nitrogen atom (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), the synthesis becomes practically infeasible. Here, we show the very first examples of a successful Mallory reaction on stilbene-like imine precursors involving the molecularly curved corannulene nucleus. The isolated yields exceed 90% and the resulting single and double aza[4]helicenes exhibit adjustable high affinity for electrons.
N), the synthesis becomes practically infeasible. Here, we show the very first examples of a successful Mallory reaction on stilbene-like imine precursors involving the molecularly curved corannulene nucleus. The isolated yields exceed 90% and the resulting single and double aza[4]helicenes exhibit adjustable high affinity for electrons.


 Please wait while we load your content...
Please wait while we load your content...