Lipase mimetic cyclodextrins†
Abstract
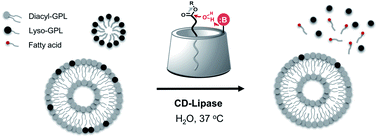
Glycerophospholipids (GPLs) perform numerous essential functions in biology, including forming key structural components of cellular membranes and acting as secondary messengers in signaling pathways. Developing biomimetic molecular devices that can detect specific GPLs would enable modulation of GPL-related processes. However, the compositional diversity of GPLs, combined with their hydrophobic nature, has made it challenging to develop synthetic scaffolds that can react with specific lipid species. By taking advantage of the host–guest chemistry of cyclodextrins, we have engineered a molecular device that can selectively hydrolyze GPLs under physiologically relevant conditions. A chemically modified α-cyclodextrin bearing amine functional groups was shown to hydrolyze lyso-GPLs, generating free fatty acids. Lyso-GPLs are preferentially hydrolyzed when part of a mixture of GPL lipid species, and reaction efficiency was dependent on lyso-GPL chemical structure. These findings lay the groundwork for the development of molecular devices capable of specifically manipulating lipid-related processes in living systems.


 Please wait while we load your content...
Please wait while we load your content...