Understanding the metal free alginate gelation process†
Abstract
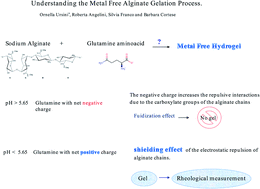
Alginate is a natural polysaccharide that has been recently gaining increasing attention as a biomaterial in the field of tissue engineering due to its favourable biocompatibility and gelation properties. Alginate hydrogels are commonly made by ionic crosslinking in the presence of divalent cations. Only a few studies have attempted to prepare alginate hydrogels without the presence of metal cations. Here the formation of metal free alginate hydrogels in the presence of the amino-acid glutamine is investigated. The transition from sol to gel is monitored by rheological measurements in the viscoelastic regime that reveal how the charged or neutral form of glutamine induces deep differences in the gelling ability. In particular, we show that the storage, G′, and loss, G′′, moduli differ significantly by shifting the glutamine zwitterionic equilibrium. Protonated amino acid could induce a shielding effect of the electrostatic repulsion of the alginate chains. Stable gels are obtained in the presence of a larger amount of free organic acid that gives rise to chain crosslink junctions and chain–chain stabilization. This opens up the possibility of preparing metal-free alginate hydrogels based on amino acid equilibria being pH sensitive.


 Please wait while we load your content...
Please wait while we load your content...