Effect of hydroxyl on antioxidant properties of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one to scavenge free radicals†
Abstract
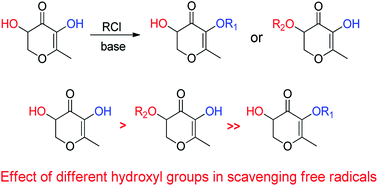
It is well known that 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) is usually formed in the Maillard reaction and it contributes to the antioxidant properties of Maillard reaction intermediates. A series of hydroxyl group protected DDMP derivatives were synthesized to further understand the source of antioxidant activity. Antioxidant abilities of the DDMP derivatives were evaluated by scavenging the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) cationic radical (ABTS˙+), 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH), and galvinoxyl radical, respectively. It was found that the introduction of protecting groups to the free hydroxyl groups of DDMP decreases their reducing abilities. In particular, the hydroxyl group at the olefin position exhibited a remarkable impact on the antioxidant activity of DDMP, indicating that the unstable enol structure in the DDMP moiety is the key factor for its antioxidant activity.


 Please wait while we load your content...
Please wait while we load your content...