Synthesis and characterization of new fluorescent boro-β-carboline dyes†
Abstract
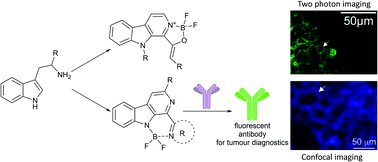
The first representatives of the new fluorescent boro-β-carboline family were synthesized by the insertion of the difluoroboranyl group into the oxaza or diaza core. The resulting compounds showed good photophysical properties with fine Stokes-shifts in the range of 38–85 nm with blue and green emission. The energetics of the excitation states and molecular orbitals of two members were investigated by quantum chemical computations suggesting effects for the improved properties of diazaborinino-carbolines over oxazaborolo-carbolines. These properties nominated this chemotype as a new fluorophore for the development of fluorescent probes. As an example, diazaborinino-carbolines were used for the specific labeling of anti-Her2 antibody trastuzumab. The fluorescent conjugate showed a high fluorophore-antibody ratio and was confirmed as a useful tool for labeling and confocal microscopy imaging of tumour cells in vitro together with the ex vivo two-photon microscopy imaging of tumour slices.


 Please wait while we load your content...
Please wait while we load your content...