Ring-opening hydrolysis of spiro-epoxyoxindoles using a reusable sulfonic acid functionalized nitrogen rich carbon catalyst†
Abstract
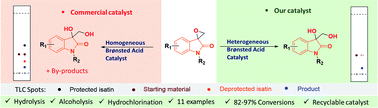
Controlling the product selectivity of a ring-opening hydrolysis reaction remains a great challenge with mineral acids and to an extent with homogeneous catalysts. In addition, even trace amounts of metal impurities in a bioactive product hinder the reaction progress. This has necessitated the development of robust and metal-free catalysts to offer an alternative sustainable route. We report a nitrogen-rich sulfonated carbon as a catalyst derived from an inexpensive precursor for the synthesis of bioactive vicinal diols of spiro-oxindole derivatives. The well-characterized catalyst shows wide generality with different electronic and steric substituents in the substrates under mild reaction conditions. Hot filtration test confirms no leaching of the acid moiety and the catalyst could be reused for four cycles with retention of activities.


 Please wait while we load your content...
Please wait while we load your content...