A comparison study of MnO2 and Mn2O3 as zinc-ion battery cathodes: an experimental and computational investigation†
Abstract
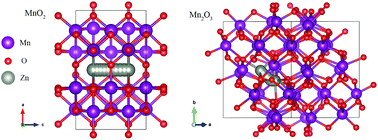
The high specific capacity, low cost and environmental friendliness make manganese dioxide materials promising cathode materials for zinc-ion batteries (ZIBs). In order to understand the difference between the electrochemical behavior of manganese dioxide materials with different valence states, i.e., Mn(III) and Mn(IV), we investigated and compared the electrochemical properties of pure MnO2 and Mn2O3 as ZIB cathodes via a combined experimental and computational approach. The MnO2 electrode showed a higher discharging capacity (270.4 mA h g−1 at 0.1 A g−1) and a superior rate performance (125.7 mA h g−1 at 3 A g−1) than the Mn2O3 electrode (188.2 mA h g−1 at 0.1 A g−1 and 87 mA h g−1 at 3 A g−1, respectively). The superior performance of the MnO2 electrode was ascribed to its higher specific surface area, higher electronic conductivity and lower diffusion barrier of Zn2+ compared to the Mn2O3 electrode. This study provides a detailed picture of the diversity of manganese dioxide electrodes as ZIB cathodes.


 Please wait while we load your content...
Please wait while we load your content...