Design, synthesis and evaluation of novel indole-2-carboxamides for growth inhibition of Mycobacterium tuberculosis and paediatric brain tumour cells†
Abstract
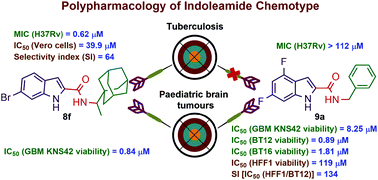
The omnipresent threat of tuberculosis (TB) and the scant treatment options thereof necessitate the development of new antitubercular agents, preferably working via a novel mechanism of action distinct from the current drugs. Various studies identified the mycobacterial membrane protein large 3 transporter (MmpL3) as the target of several classes of compounds, including the indole-2-caboxamides. Herein, several indoleamide analogues were rationally designed, synthesised, and evaluated for their antitubercular and antitumour activities. Compound 8g displayed the highest activity (MIC = 0.32 μM) against the drug-sensitive (DS) Mycobacterium tuberculosis (M. tb) H37Rv strain. This compound also exhibited high selective activity towards M. tb over mammalian cells [IC50 (Vero cells) = 40.9 μM, SI = 128], suggesting its minimal cytotoxicity. In addition, when docked into the MmpL3 active site, 8g adopted a binding profile similar to the indoleamide ligand ICA38. A related compound 8f showed dual antitubercular (MIC = 0.62 μM) and cytotoxic activities against paediatric glioblastoma multiforme (GBM) cell line KNS42 [IC50 (viability) = 0.84 μM]. Compound 8f also showed poor cytotoxic activity against healthy Vero cells (IC50 = 39.9 μM). Compounds 9a and 15, which were inactive against M. tb, showed potent cytotoxic (IC50 = 8.25 and 5.04 μM, respectively) and antiproliferative activities (IC50 = 9.85 and 6.62 μM, respectively) against KNS42 cells. Transcriptional analysis of KNS42 cells treated with compound 15 revealed a significant downregulation in the expression of the carbonic anhydrase 9 (CA9) and the spleen tyrosine kinase (SYK) genes. The expression levels of these genes in GBM tumours were previously shown to contribute to tumour progression, suggesting their involvement in our observed antitumour activities. Compounds 9a and 15 were selected for further evaluations against three different paediatric brain tumour cell lines (BT12, BT16 and DAOY) and non-neoplastic human fibroblast cells HFF1. Compound 9a showed remarkable cytotoxic (IC50 = 0.89 and 1.81 μM, respectively) and antiproliferative activities (IC50 = 7.44 and 6.06 μM, respectively) against the two tested atypical teratoid/rhabdoid tumour (AT/RT) cells BT12 and BT16. Interestingly, compound 9a was not cytotoxic when tested against non-neoplastic HFF1 cells [IC50 (viability) = 119 μM]. This suggests that an indoleamide scaffold can be fine-tuned to confer a set of derivatives with selective antitubercular and/or antitumour activities.


 Please wait while we load your content...
Please wait while we load your content...