Recent progress in application of nanocatalysts for carbonylative Suzuki cross-coupling reactions
Abstract
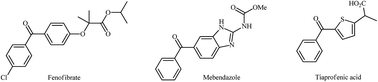
In the past few decades, cross-coupling of aryl halides and arylboronic acids in the presence of carbon monoxide (CO), also called carbonylative Suzuki coupling, to form two new carbon–carbon bonds in the production of synthetically and biologically important biaryl ketones, has been widely studied. Consequently, various catalytic systems have been extensively investigated in order to maximize the efficiency of this appealing area of biaryl ketone synthesis. As evidenced in the literature, nanometal-based systems are among the most powerful catalysts for this transformation as their large surface area to volume ratio and reactive morphologies allow faster reaction rates under milder CO pressure even at very low catalyst loadings. This review aims to provide an overview of the recent advances and achievements in the application of nano-sized metal catalysts for carbonylative Suzuki cross-coupling reactions, which may serve as an inspiration to researchers in their future work.
- This article is part of the themed collection: 2021 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...