Arenesulfonyl indole: new precursor for diversification of C-3 functionalized indoles
Abstract
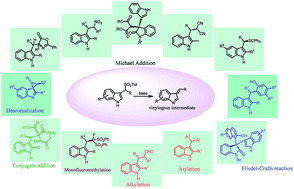
Arenesulfonyl indoles, bearing a good leaving group, are effective precursors for vinylogous imine intermediates which are generated in situ under basic conditions. This intermediate can readily react with other nucleophilic reagents to obtain C-3 substituted indole derivatives. In the last few years, a plethora of exciting synthetic applications of this substrate have been exploited. The stability of arylsulfonyl-containing substrates, mild reaction conditions, and the large variety of nucleophiles involved in these procedures are the key to their success in organic synthesis.
- This article is part of the themed collection: 2021 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...