High efficiency removal of Pb(ii) in aqueous solution by a biochar-supported nanoscale ferrous sulfide composite
Abstract
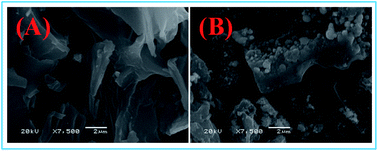
A biochar-supported nanoscale ferrous sulfide composite was prepared and applied for the treatment of Pb(II) ions in aqueous solution. The experimental results of SEM, EDS, XRD, and FT-IR spectroscopy clearly implied that the biochar was successfully modified with nanoscale ferrous sulfide composite. The maximum adsorption capacity of Pb(II) ions by FeS@biochar reached 88.06 mg g−1. Compared with other reported adsorbents, the removal rate of Pb(II) ions by FeS@biochar was higher. The pseudo-second-order kinetic model and Langmuir isotherm model could better fit the experimental adsorption results. The removal rate of Pb(II) ions by FeS@biochar was controlled by the chemical reaction and monolayer adsorption on the surface of FeS@biochar. The mechanisms of Pb(II) removal from aqueous solutions by biochar involved electrostatic attraction, hydrogen bonding, physical adsorption, ion exchange, and chemical precipitation. Additionally, the chemical stability and reusability of FeS@biochar were good. It is also an environment-friendly material for low-cost wastewater treatment.


 Please wait while we load your content...
Please wait while we load your content...