Electrochemically facilitated oxidative C–H amination enables access to fluorescent N9-fused tricyclic xanthines†
Abstract
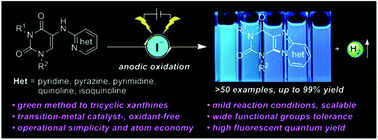
In this study, an environmentally benign electrochemical protocol for intramolecular oxidative amination is developed, which offers an efficient and sustainable route to access diverse fused tricyclic systems of pyrido[1,2-e]purine-2,4-dione derivatives with pharmacological interest, exhibiting broad scope and generality (>50 examples, yields up to 99%). This electrochemical strategy features good functional group tolerance and permits access to compounds that were previously not achievable under thermal conditions. The photoluminescence properties of the representative N9-fused tricyclic xanthines have also been investigated.


 Please wait while we load your content...
Please wait while we load your content...