An azo-bridged ring system enabled by-standing immobilization of a chiral diene ligand†
Abstract
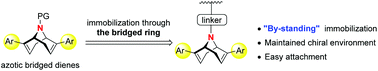
A family of 9-azabicyclo[3.3.1]nonadienes were designed, prepared and successfully applied in the rhodium-catalyzed addition of arylboronic acids to N-tosylarylimines, affording chiral diarylmethyl amides with excellent enantioselectivities (up to 97% ee). The nitrogen atom in the bridged ring enabled a facile immobilization of diene ligands to silica. The obtained heterogeneous catalyst was evaluated in the above addition reaction and reused five times without significant loss in yield and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...