Direct access to α-acyloxycarbonyl compounds and esters via oxidative esterification of aldehydes under visible light†‡
Abstract
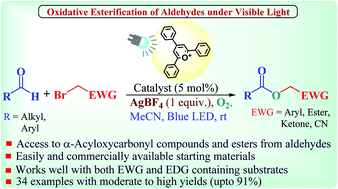
Herein we report the efficient synthesis of α-acyloxycarbonyl compounds and esters starting directly from aldehydes and α-bromocarbonyl compounds/benzyl bromide derivatives and bromo nitriles using an organic photocatalyst in combination with silver tetrafluoroborate under visible light at room temperature. The reaction is compatible with a variety of functional groups and has been successfully applied to the synthesis of various molecules. The strength of this protocol was effectively demonstrated by utilising both aromatic and aliphatic aldehydes. The detailed controlled studies including the labeling experiment and fluorescence spectroscopic data clearly revealed the reductive quenching of the photoredox catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...