Nickel-catalyzed Suzuki–Miyaura cross-coupling of C–F bonds†
Abstract
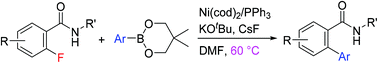
The efficient Suzuki–Miyaura cross-coupling of ortho-fluoro aromatic secondary amides with aryl boronates is described. The use of KOtBu is essential for the reaction to proceed. The function of the base is to abstract a proton from an amide nitrogen to generate a weak conjugate base, such as an amidate, which functions as a directing group. The reaction proceeds effectively, even at 60 °C. The reaction exhibits a good tolerance for functional groups and a broad scope for aromatic amides.


 Please wait while we load your content...
Please wait while we load your content...