Dehydrogenative 6π heterocyclization under visible light irradiation and mechanistic insights†
Abstract
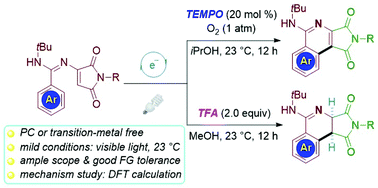
A visible-light-driven oxidative 6π heterocyclization for the synthesis of structurally diverse π-conjugated polycyclic 1-aminoisoquinolines has been developed. The reaction proceeds under visible-light or sunlight, obviates the need for photocatalysts and transition-metals, and features an unusually broad substrate scope and high efficacy. This synthetic pathway provided an easy access to highly fluorescent small molecules with high photoluminescence quantum yields. The N-heterocycles exhibit suitable optical properties for application as fluorescence quantum yield standards. DFT calculations were employed to gain insight into the mechanism and the results show that deprotonation is the rate-determining step, which can be promoted by a TFA additive.


 Please wait while we load your content...
Please wait while we load your content...