Regioselective synthesis of indenones via nickel-catalyzed Larock annulations†
Abstract
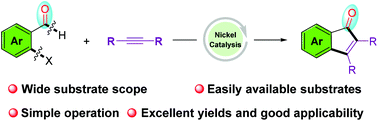
We present herein highly effective nickel-catalyzed Larock annulations of substituted 2-formylphenyl trifluoromethanesulfonate with alkynes to provide an access to a wide range of indenones that are valuable synthetic intermediates for natural compounds. This catalytic system is shown to proceed through a redox neutral arylation to produce indenone products in high yields with excellent regioselectivities. In addition, a highly efficient transformation of the natural product estrone via Ni-catalyzed Larock annulation can also be achieved.


 Please wait while we load your content...
Please wait while we load your content...