Photocatalyzed intermolecular amination for the synthesis of hydrazonamides†
Abstract
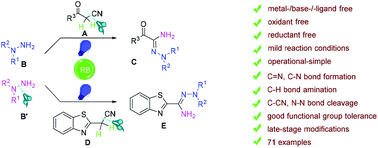
Hydrazonamides are fundamental building blocks in many pharmaceuticals and agricultural chemicals. Although previous transformations have been accomplished through multi-step reactions under harsh conditions, developing highly efficient synthetic strategies remains challenging. Considering the structural characteristic of hydrazonamides, a sequential multi-component reaction of β-ketonitriles with N,N-disubstituted hydrazines is designed and developed through a photocatalyzed intermolecular amination process. This work reports the first example of the use of N,N-disubstituted hydrazines as two different “amine” sources, characterized by isotope labeling experiments. The C–CN/N–N bonds are cleaved and new C–N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds are constructed in a one-pot reaction. This protocol can be carried out without the addition of any external metals, ligands, bases, oxidants and reductants, and possesses the advantages of operational simplicity, mild reaction conditions, and good functional group tolerance. Furthermore, this approach enables late-stage modifications of structurally complex bioactive molecules, natural products and drugs, thus showing potential applications in the field of organo-pharmaceutical chemistry.
N bonds are constructed in a one-pot reaction. This protocol can be carried out without the addition of any external metals, ligands, bases, oxidants and reductants, and possesses the advantages of operational simplicity, mild reaction conditions, and good functional group tolerance. Furthermore, this approach enables late-stage modifications of structurally complex bioactive molecules, natural products and drugs, thus showing potential applications in the field of organo-pharmaceutical chemistry.


 Please wait while we load your content...
Please wait while we load your content...