Strategies towards endo-type B polycyclic polyprenylated acylphloroglucinols: total synthesis of regio-hyperibone L and (+)-epi-clusianone†
Abstract
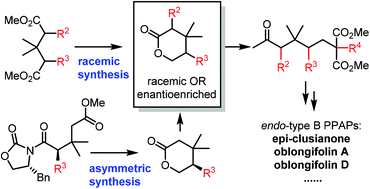
A concise and stereoselective method toward the divergent synthesis of polycyclic polyprenylated acylphloroglucinols and the analogues in both racemic and asymmetric fashions was developed. With the bioinspired Me2AlSEt-promoted domino Dieckmann cyclization as the key strategy, the total synthesis of regio-hyperibone L and the first asymmetric total synthesis of natural epi-clusianone were achieved, which determined the absolute configuration of epi-clusianone and demonstrated the broad synthetic applicability of the domino method. Furthermore, by a stereoselective alkylation/reductive deprotection/cyclization strategy, the key enantioenriched 5-substituted lactones for accessing epi-clusianone are prepared efficiently, which provides a general asymmetric approach towards the synthesis of endo-type B PPAPs.


 Please wait while we load your content...
Please wait while we load your content...