Copper-catalyzed enantioselective arylboronation of activated alkenes leading to chiral 3,3′-disubstituted oxindoles†
Abstract
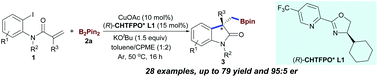
We here describe the first example of copper-catalyzed enantioselective arylboronation of activated alkenes with bis(pinacolato)diboron (B2Pin2) for the synthesis of chiral 3,3′-disubstituted oxindoles. This method enables the formation of a C(sp2)–C(sp3) bond and a C(sp3)–B bond in a single operation, and provides a new asymmetric synthetic toolbox to incorporate boron atoms into heterocycle motifs using inexpensive copper catalysis.


 Please wait while we load your content...
Please wait while we load your content...