Stereoselective synthesis of α-d-fructofuranosides using a 4,6-O-siloxane-protected donor†
Abstract
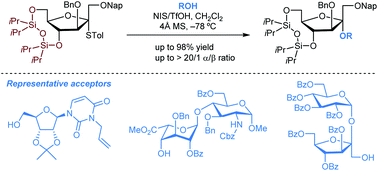
A stereoselective glycosylation approach to α-D-fructofuranosides employing a 4,6-O-tetraisopropyldisiloxanylidene-containing thio-fructofuranoside donor is described. This method features broad substrate scope and good functional-group tolerance, which allowed the efficient synthesis of the α-isomers of inulin oligosaccharides.


 Please wait while we load your content...
Please wait while we load your content...