An organophotoredox-catalyzed redox-neutral cascade involving N-(acyloxy)phthalimides and maleimides†
Abstract
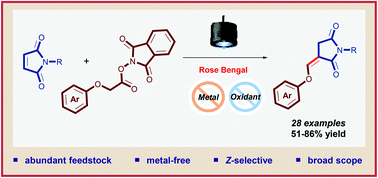
An organophotoredox-catalyzed reduction/addition/oxidation cascade of N-protected maleimides and N-(acyloxy)phthalimides is documented. The mild and efficient redox-neutral process involves the hitherto unknown Giese-type addition of aryloxy-alkyl radicals on N-protected maleimides and a successive oxidation allowing an overall Z-alkenylation of the N-substituted pyrrolidine-2,5-dione motif through a formal translocation of the maleimide double bond.


 Please wait while we load your content...
Please wait while we load your content...