A one-pot stepwise approach to axially chiral quinoline-3-carbaldehydes enabled by iminium–allenamine cascade catalysis†
Abstract
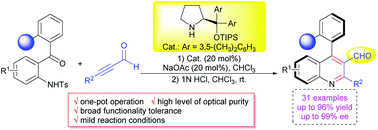
An unprecedented atroposelective annulation between 2-(tosylamino)aryl ketones and 2-alkynals for the construction of N-heterobiaryl atropisomers is achieved. The reaction involves a Michael/aldol cascade reaction catalyzed by a chiral secondary amine organocatalyst followed by acid promoted aromatization, providing a wide spectrum of axially chiral 4-arylquinoline-3-carbaldehydes in a one-pot fashion in good to high yields (up to 96%) and with excellent enantiocontrol (up to 99% ee). The power of the approach is also demonstrated by versatile transformations towards other functional N-heterobiaryl atropisomers.


 Please wait while we load your content...
Please wait while we load your content...