Transition-metal-free synthesis of 5-amino-1,2,3-triazoles via nucleophilic addition/cyclization of carbodiimides with diazo compounds†
Abstract
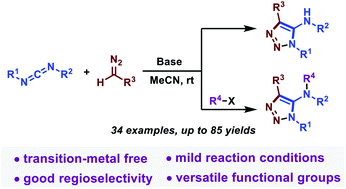
A simple and transition-metal-free strategy to construct 5-amino-1,2,3-triazoles using carbodiimides and diazo compounds has been developed. This protocol involves a cascade nucleophilic addition/cyclization process and is accomplished under mild conditions. Further functionalization enriched the molecular diversity of triazoles. Control experiments and DFT calculations clarify the reaction mechanism and rationalize the kinetic and thermodynamic selectivity observed in the transformation. The late-stage derivatization and gram-scale synthesis reveal the promising utility of this methodology.


 Please wait while we load your content...
Please wait while we load your content...