Polybenzoxazines: a sustainable platform for the design of fast responsive and catalyst-free vitrimers based on trans-esterification exchanges†
Abstract
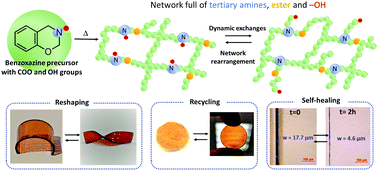
This work explores a new strategy, aiming for the synthesis of catalyst-free vitrimers by taking advantage of the abundant number of tertiary amines covalently bound into a polybenzoxazine network. A bio-based monomer was obtained by reacting 4,4-bis(4-hydroxyphenyl)valeric acid, polyethylene glycol, paraformaldehyde and mono-ethanolamine, via consecutive solvent-free Fischer esterification and Mannich-like ring-closure. The two-step reaction led to the formation of quadri-telechelic benzoxazine-terminated polyethylene glycol monomers, containing ester bonds and aliphatic hydroxyl groups. The structural features of the resulting products were substantiated by 1H NMR, 13C NMR, elemental analysis, and FTIR. The occurrence of the thermally induced ring-opening polymerization was monitored by rheological measurements and DSC. At 140 °C, the monomers show a short gelation time (145 seconds). Once polymerized, the polybenzoxazine exhibits a relatively high temperature of α mechanical relaxation (93 °C). Due to the ability of tertiary amines to catalyze transesterification reactions, and to the abundant number of hydroxyl groups, the material enables exchange reactions without the use of an external catalyst. It possesses all the typical characteristics of a vitrimer, such as recycling, reshaping, and self-healing. Short stress-relaxation times were measured (116 s at 170 °C). Finally, the effect of the structural features of the vitrimer was investigated by tuning the crosslinking density of the network and the number of hydroxyl groups, shedding more light on the mechanism of self-catalysis and the range of properties. Therefore, such a strategy constitutes an efficient and versatile route for an easy elaboration of mono-component, catalyst-free, and fast responsive vitrimers.


 Please wait while we load your content...
Please wait while we load your content...