Manganese(iii)-promoted thiocarbonylation of alkylborates with disulfides: synthesis of aliphatic thioesters†
Abstract
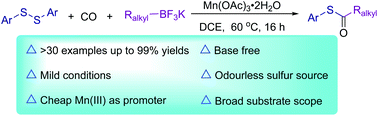
A Mn(III)-promoted thiocarbonylation procedure toward the synthesis of thioesters has been developed. By employing easily available disulfides and potassium alkyltrifluoroborates as the starting materials, and cheap and non-toxic Mn(OAc)3·2H2O as the promotor, a broad range of thioesters were synthesized in good to excellent yields via radical intermediates.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...