A Chan–Evans–Lam approach to trisubstituted vinyl ethers†
Abstract
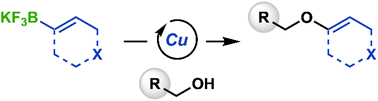
Trisubstituted vinyl ethers were accessed via Chan–Evans–Lam coupling of vinyl trifluoroborates and primary aliphatic alcohols. This approach complements prior methods that required the use of neat liquid alcohol coupling partners. A palladium-catalyzed redox-relay Heck reaction was used to convert several vinyl ethers into aldehyde-functionalized 1,3-dihydroisobenzofurans.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...