Photophysics, photochemistry and bioimaging application of 8-azapurine derivatives†
Abstract
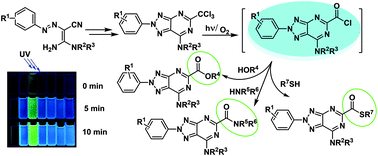
New 2-aryl-1,2,3-triazolopyrimidines were designed, synthesized, and characterized. Their optical properties were thoroughly studied in the solid phase, in solution and in a biological environment. Density Functional Theory (DFT) based calculations were performed, including the molecular geometry optimization for both the ground state and the first singlet excited state, the prediction of the UV-Vis absorption and fluorescence spectra, the determination of the molecular electrostatic properties and the solvent effect on the optical properties. The emission intensity was revealed to increase in time upon irradiation. Mass spectrometric research, quantum mechanical calculations, and analysis of literature data suggested a possible photo-transformation pathway through the homolytic cleavage of one of the C–Cl bonds upon irradiation with UV light. The structure of the active intermediate was identified by the series of mass spectrometry experiments and via synthesis of putative transformation products. The kinetic parameters measured in different solvents allowed estimating the rate of these photo-transformations. Biological experiments demonstrated that 2-aryl-1,2,3-triazolopyrimidines penetrate cells and selectively accumulate in the cell membrane and the Golgi complex and endoplasmic reticulum. Their unique properties pave the way for new possible applications of fluorescent 8-azapurines in biology and medicine.


 Please wait while we load your content...
Please wait while we load your content...