Palladium-catalyzed difunctionalization/dearomatization of N-benzylacrylamides with α-carbonyl alkyl bromides: facile access to azaspirocyclohexadienones†
Abstract
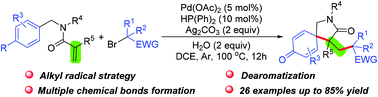
An efficient palladium-catalyzed difunctionalization/dearomatization of N-benzylacrylamides with α-carbonyl alkyl bromides as alkyl radical precursors has been described. Various α-carbonyl alkyl bromides, including α-bromoalkyl esters and ketones, reacted smoothly to provide important azaspirocyclohexadienones in moderate to excellent yields. In addition, mechanistic studies suggested that the reaction proceeded via a radical pathway.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...